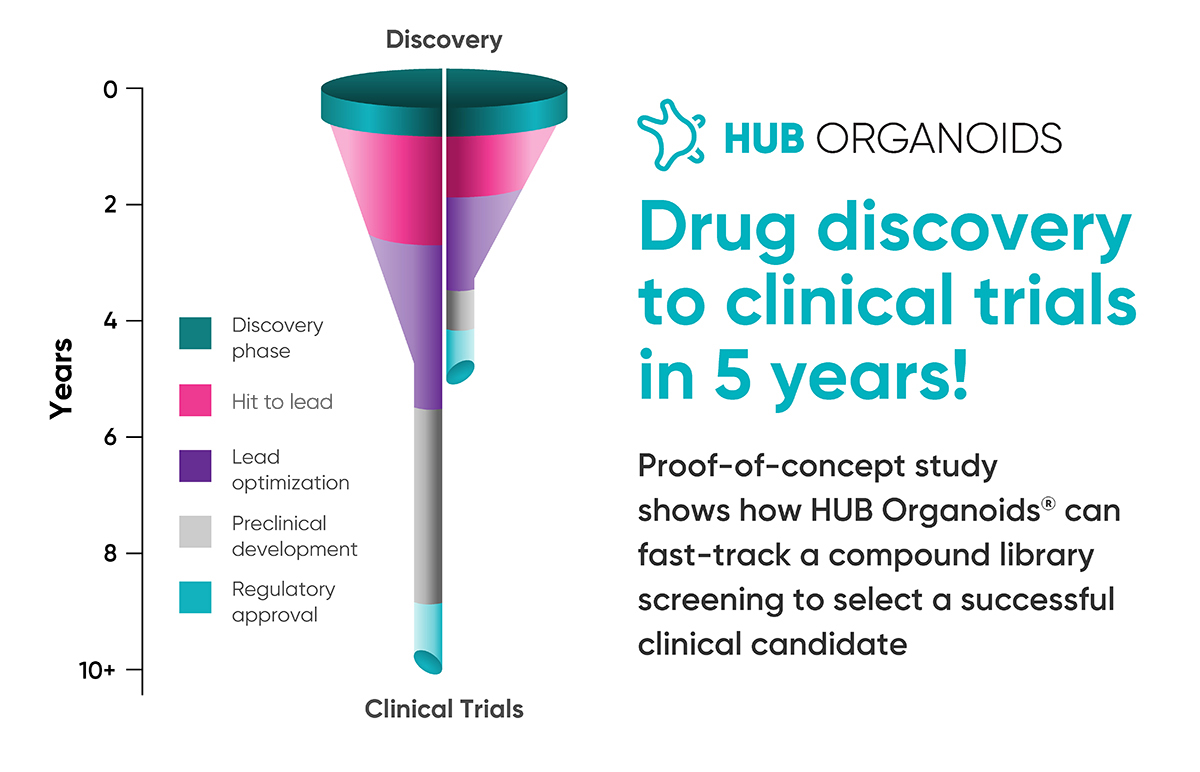
From ten to five years: The journey of a drug that makes it to the clinical trial with HUB Organoids.
Patients with mutated KRAS (Kirsten rat sarcoma virus) gene account for around 30-50% of the population with colorectal cancer (CRC) and do not respond to cetuximab, a recognized choice of antibody therapy. A new proof-of-concept study led by the SuppresSTEM European consortium discovers a bispecific antibody that prevents the onset of metastasis and sedates the growth of primary tumors in experimental models of colorectal cancer, including patient-derived organoids. The study published in Nature Cancer represents the first peer-reviewed published work in the oncology field that demonstrates the feasibility of using HUB Organoids to screen a library of compounds and progress a lead agent from early-stage discovery to clinical trials. Notably, the development of the lead agent was accomplished in about five years, a significantly shorter timeframe than a classic drug discovery and development pipeline.
HUB Organoids are stem cell-derived mini-organs developed from patient resected or biopsied tissue that can be grown in the laboratory. With their ability to retain the patient tumor’s key genetic and physiological characteristics, cancer organoids have been gradually accepted as a better alternative to canonical systems such as cell lines or animal models.
A needle in a haystack
It takes 10-15 years and an average of US$1billion to develop a successful drug. The stats illustrate high attrition, where only 5% of all promising targets from the preclinical phase make it to the manufacturing phase of the drug development pipeline. Experts attribute this high failure rate to the over-reliance on animal models or cell lines that only partially recapitulate the patients’ genetic and physiological profile leading to candidate agents with marginal clinical efficacy and high toxicity risk.
This study leverages the potential of patient-derived organoid biobanks to screen a library of hundreds of bispecific antibodies. Then, it identifies a bispecific antibody that binds to the stem cell marker Leucine-rich repeat-containing G-protein coupled Receptor 5 (LGR5) and the tyrosine kinase receptor Epithelial Growth Factor Receptor (EGFR) on cancer stem cells and robustly blocks the growth of both KRAS-wild type and KRAS-mutant CRC organoids. It also validates the therapeutic potential in a clinical setting by reporting no toxicity effects on normal tissue-derived HUB Organoids from the same patient, thus demonstrating that the new antibody can effectively suppress tumor growth without affecting healthy tissue. Furthermore, the study shows that the newly developed bispecific antibody reduces tumor size and it is also capable of dampening metastasis formation when CRC organoids are engrafted into mouse models to derive patient-derived organoid xenografts (PDXO).
This promising new agent is currently being evaluated on patients in clinical trials. The preliminary reports from the Phase I dose-escalation studies indicate that the antibody is well tolerated by patients with manageable infusion-related reactions and has shown promising results in five head and neck cancer patients reducing tumor size. (ClinicalTrials.gov identifier: NCT03526835).
This five-year-long work represents a critical advance in oncology and stem cell research. It opens a portal of accelerated drug discovery and development in a scalable, economical, and clinically relevant fashion.
To read more, click here.
Want to use HUB Organoids for your research? Contact us.
References
Herpers, B., Eppink, B., James, M. I., Cortina, C., Cañellas-Socias, A., Boj, S. F., . Throsby, M. (2022). Functional patient-derived organoid screenings identify MCLA-158 as a therapeutic EGFR × LGR5 bispecific antibody with efficacy in epithelial tumors. Nature Cancer, 3(4), 418-436. doi:10.1038/s43018-022-00359-0
Verduin, M., Hoeben, A., De Ruysscher, D., & Vooijs, M. (2021). Patient-derived cancer organoids as predictors of treatment response. Frontiers in Oncology, 11. doi:10.3389/fonc.2021.641980




