Organoids derived from patients accurately mirror the physiology and genetic makeup of the source tissue, offering a reliable platform for evaluating the toxicity of drugs in the preclinical phase. Our sophisticated culture methods facilitate the combination of immune cells and stromal elements in a co-culture system, accurately simulating the tissue’s microenvironment complexity.
Regulatory requirements demand mammalian safety testing, but choosing the right species is crucial to unveil potential human toxicities. Our advanced organoid-based GI toxicology platform allows you to test for unwanted side effects across multiple common regulatory-approved animal species and to compare these results with human-derived organoids. This allows you to make an educated selection for further in vivo safety testing.
Preclinical animal studies can often reveal discordant toxicity profiles, dependent on species, requiring further investigation to understand relevance to humans. Our innovative in vitro GI toxicology platform offers a solution to bridge the gap between preclinical and human data. By comparing your drug’s effects on human organoids and animal species from your preclinical studies, you can gain valuable insights into its translatability to humans. Furthermore, it can also facilitate the investigation into the mechanisms of toxicity, thereby contributing to the development of improved second-generation drug candidates or the formulation of innovative strategies to reduce toxicity.


Anticipating human reactions at an early stage can enhance the safety of drug candidates, diminish the need for animal testing, and potentially save billions by improving R&D productivity. Our approach facilitates the early detection of off-target toxicity during the preclinical stage. Our comprehensive biobank, housing human organoids from hundreds of patients, allows for simultaneous evaluation of your drug candidates on both diseased and healthy organoids derived from the same donor. This powerful tool empowers you to predict both on-target and off-target toxicity early in development, enabling informed decisions on which compounds to advance further.

ON-DEMAND SLIDES
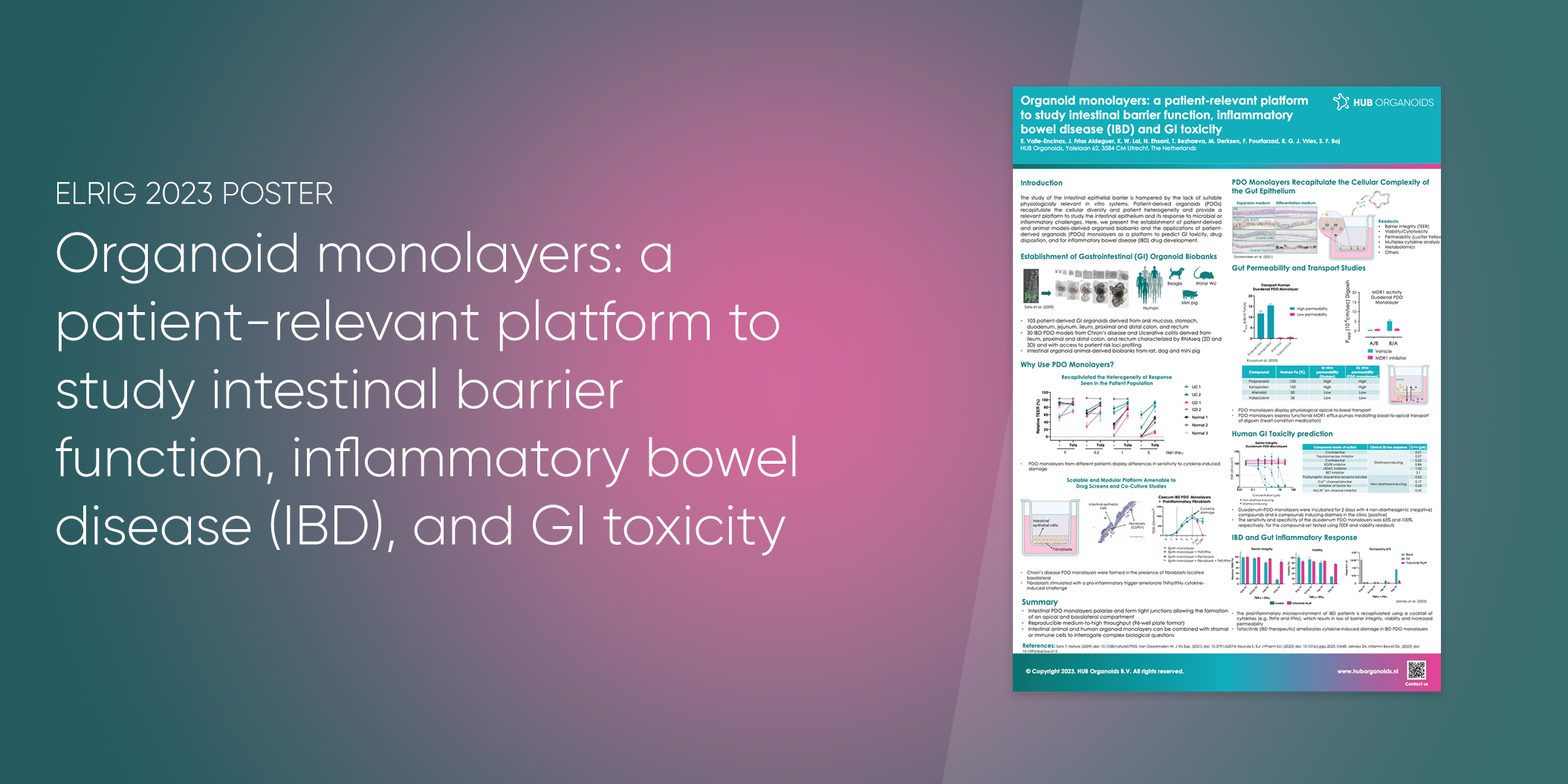
POSTER