Explore a novel 3D co-culture model of inflammatory bowel disease (IBD) using patient-derived organoids (PDOs) and fibroblasts. Learn how this system recapitulates key IBD hallmarks and facilitates drug discovery & development.


Facing poor translational relevance of cell cultures and the inability of animal models to reflect patient IBD insights, the clients approached us to develop a novel 3D organoid co-culture system that recapitulated the human intestinal microenvironment.

We proposed a physiologically relevant 3D co-culture assay, combining intestinal fibroblasts (± inflammatory stimulation) with IBD patient-derived organoids to enable dynamic signaling and direct interaction within a 3D environment. We verified our assay using the standard of care, following in-depth optimizations of all readouts and inflammation triggers, providing the client with a valuable tool for their preclinical IBD drug discovery and development.

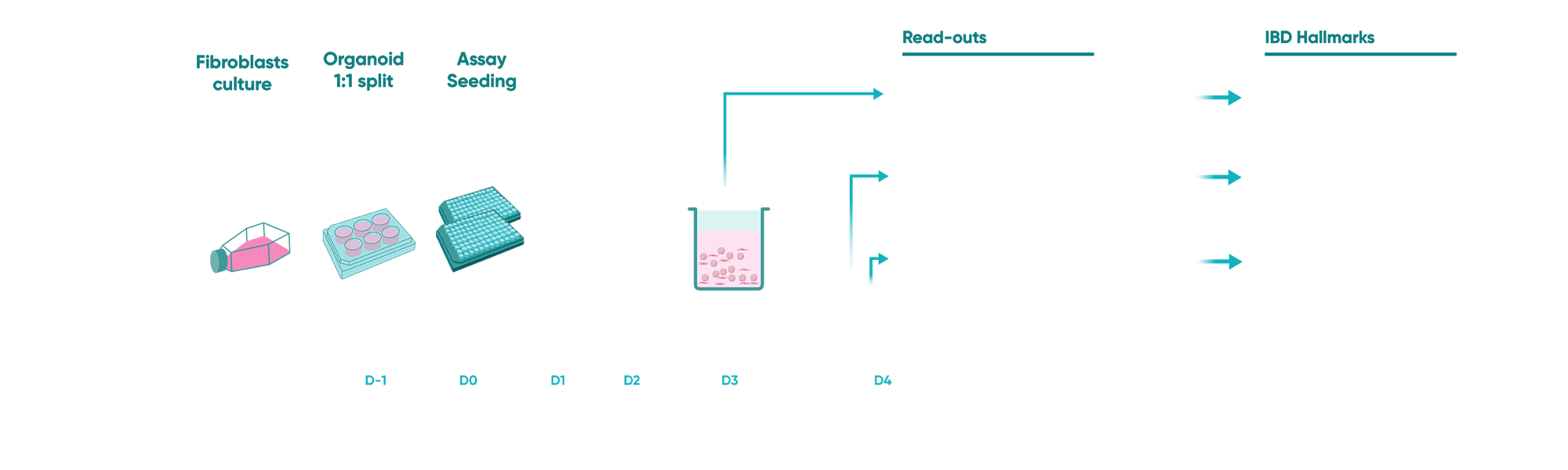
Fig 1: Schematic of the study design
Keeping our client’s research hypothesis in mind, we designed an experimental set-up that included co-culturing human fibroblasts with IBD patient-derived organoids. The study protocol included induction of an inflammatory trigger for an inflammatory fibroblast phenotype followed by a cytokine-induced epithelial damage mimicking mucosal IBD environment. In this phase, along with the client, we did an intensive literature search to understand probable readouts from the experiment that could replicate the hallmarks of IBD pathogenesis.
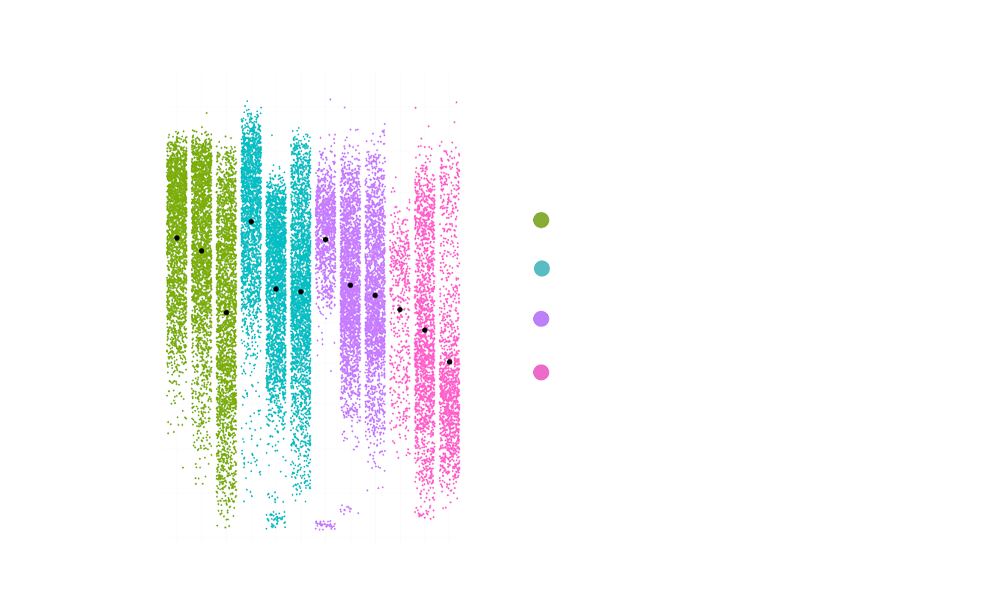
A critical first step was the establishment of a reliable and reproducible in vitro model. This involved optimizing the 3D co-culture conditions for fibroblasts and IBD patient-derived epithelial organoids. We carefully titrated the concentration of an inflammatory trigger to induce the desired inflammatory fibroblast phenotype, and performed multiple rounds of culture condition optimization to ensure the system’s robustness. Organoid swelling served as a key readout to visualize the impact of the inflammatory trigger on the epithelium. Importantly, the reproducibility of this swelling metric was rigorously assessed, achieving a Z’ factor of >0.5, demonstrating excellent assay quality and reliability for subsequent experiments.
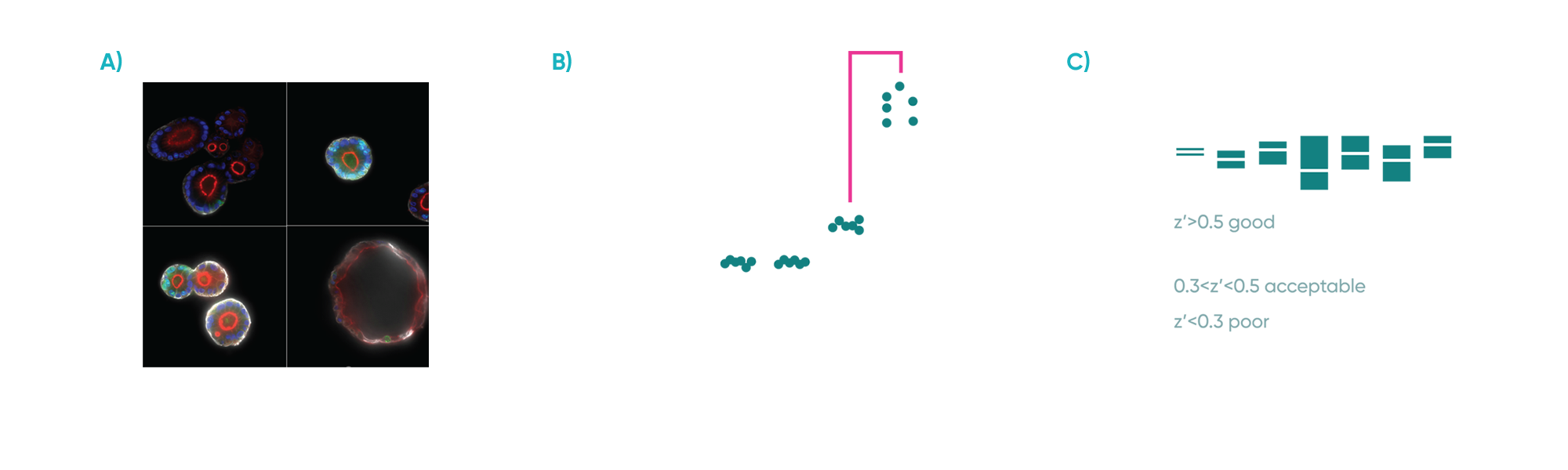
Fig 2: A) Representative confocal microscopy images of PDOs cultured alone or with fibroblasts with or without inflammatory stimuli. PDOs were stained for Phalloidin (red), KI67 (green), KRT20 (white) and DAPI (blue). B) Organoid area change in response to the presence of fibroblasts and/or inflammatory stimuli. C) Z’ scores for organoid area change of co-cultures exposed to inflammation across experiments. Each experiment included 3 plates with independent controls.
A core objective was to characterize the complex communication between inflammatory fibroblasts and epithelial cells within the co-culture. We investigated the role of various soluble mediators released by fibroblasts and the corresponding chemokine response from epithelial cells. Our data revealed that the inflammatory trigger significantly stimulated fibroblasts to induce chemokine production by the epithelial cells, highlighting a key aspect of intercellular signalling in IBD.
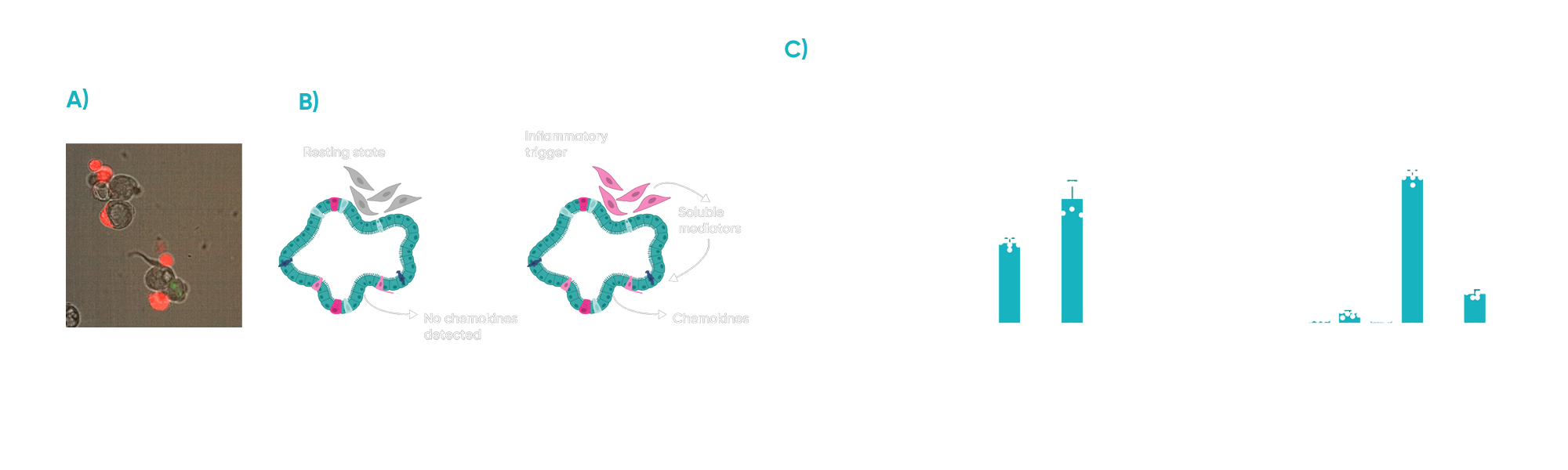
Fig 3: A) Fibroblasts (red) associate with the intestinal epithelium when co-cultured with PDOs. B) Cartoon representing the cross-talk between (proinflammatory) fibroblasts and epithelial cells. C) Inflammatory trigger drives fibroblasts to activate chemokine production on epithelial cells.
In addition to soluble mediator analysis, we examined other key hallmarks of IBD within the co-culture system. Notably, we observed that inflammatory stimuli resulted in a significant decrease in organoid cell proliferation, mirroring the impaired tissue regeneration often seen in IBD.
To further validate the translational relevance of our co-culture assay, we assessed the effect of a standard-of-care treatment for IBD. Notably, the addition of tofacitinib, a clinically relevant therapeutic, resulted in a reduction of both organoid swelling and cell death within the inflammatory co-cultures, demonstrating the assay’s utility for evaluating potential therapeutic targets and interventions.
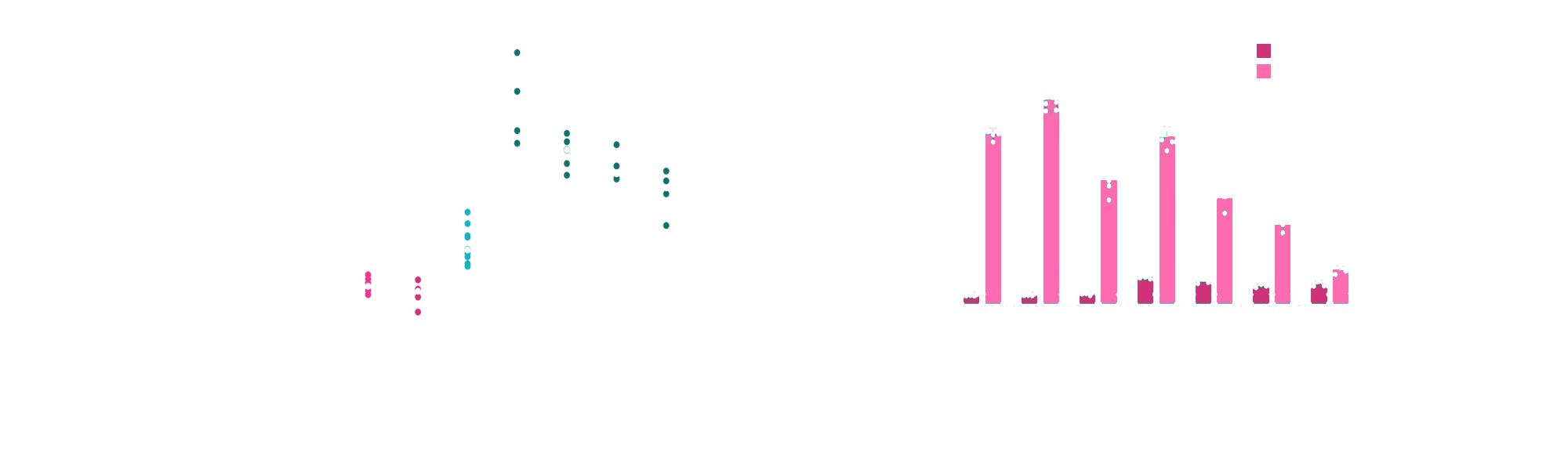
Fig 5: A) Tofacitinib limits the organoid swelling and B) cytokine-induced cell death induced by the proinflammatory fibroblasts.
In conclusion, this organoid-based co-culture system enhances our understanding of IBD pathogenesis and offers a translational tool for IBD drug discovery development. By accurately modeling key aspects of the IBD microenvironment, it can accelerate the discovery of effective treatments.