Average cost to market per drug
Average time to bring a drug to the market
of new drugs that show efficacy fail in clinical trials
Developing a new drug is extraordinarily costly, largely because traditional preclinical models – cell lines, animal studies, and other non-human systems—frequently fail to predict how patients will respond. These limitations allow weak or non-efficacious candidates to progress into clinical trials, where failures become exponentially more expensive. Phase II and III attrition now accounts for the majority of sunken R&D cost, contributing to an average $2.5B investment per approved drug and timelines that stretch a decade or more. The status quo is no longer sustainable: improving human relevance in early discovery is essential to reduce late-stage failure, protect capital, and accelerate delivery of effective therapies to patients.
Disclaimer: These figures are illustrative approximations based on industry averages; actual costs and outcomes may vary
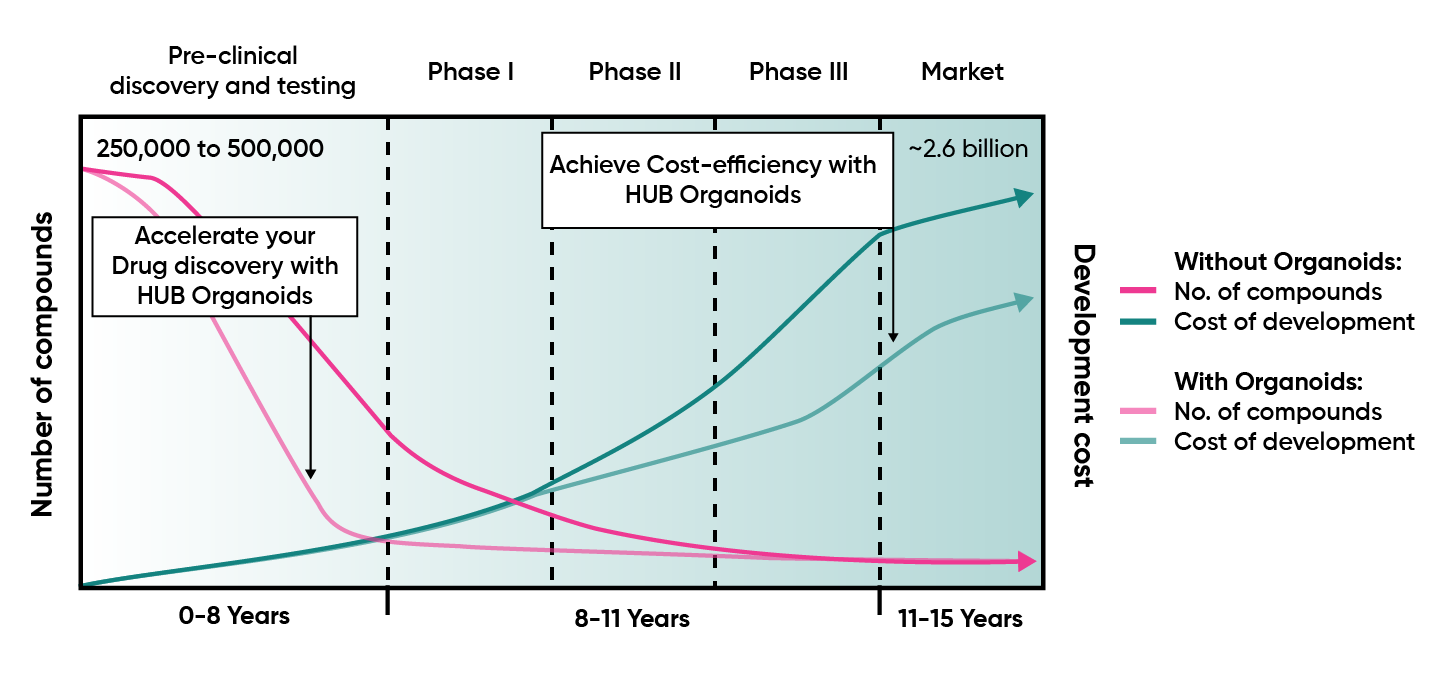
Drug development is capital intensive, with traditional models incurring ~$2.5B per approved drug and >90% failure rates in clinical trials. HUB Organoids enables a candidate prioritization paradigm – allowing you to predict success or fail fast suboptimal compunds, and empowering executive teams to reallocate capital early, optimize portfolio returns, and maximize shareholder value. With validated organoid models, companies can de-risk clinical investment, accelerate timelines, and achieve up to 40% program-level cost savings
Join the companies redefining clinical predictability
By adopting organoid technology early in development, pharma leaders are cutting late-stage attrition, strengthening portfolio capital efficiency, and increasing the odds of blockbuster approvals. Want to discuss your research program with us?