
The client, developing bispecific T cell engager (BiTE) for solid tumor indications, required a preclinical system capable of accurately assessing both efficacy and safety of their lead candidate. Conventional 2D models lacked the complexity needed to evaluate tumor-selective killing and immune engagement within a human-relevant microenvironment. Specifically, they needed a translational platform that could preserve tumor heterogeneity, reflect patient-specific antigen expression, and support functional co-culture with T cells to de-risk downstream in vivo studies and guide candidate optimization.

We applied our PDO–T cell co-culture platform to model antigen-specific immune engagement and cytotoxicity. Using matched tumor and normal non-small cell lung cancer (NSCLC) patient-derived organoids, we established co-cultures with primary T cells to assess the bispecific antibody’s ability to induce selective killing towards tumor cells and activate T cells. The system enabled head-to-head comparison of on-target efficacy and potential off-tumor toxicity, with quantitative functional readouts – providing the client with translationally predictive data ahead of in vivo studies.
We designed an experiment with 3 key assays:

Leveraging our large living biobank, we suggested testing the expression of the TAA in NSCLC tumor organoids and matched normal lung organoids. Using immunohistochemistry (IHC) (Figure 1A) and flow cytometry (Figure 1B) data, we supported our client in selecting the most relevant organoid models.
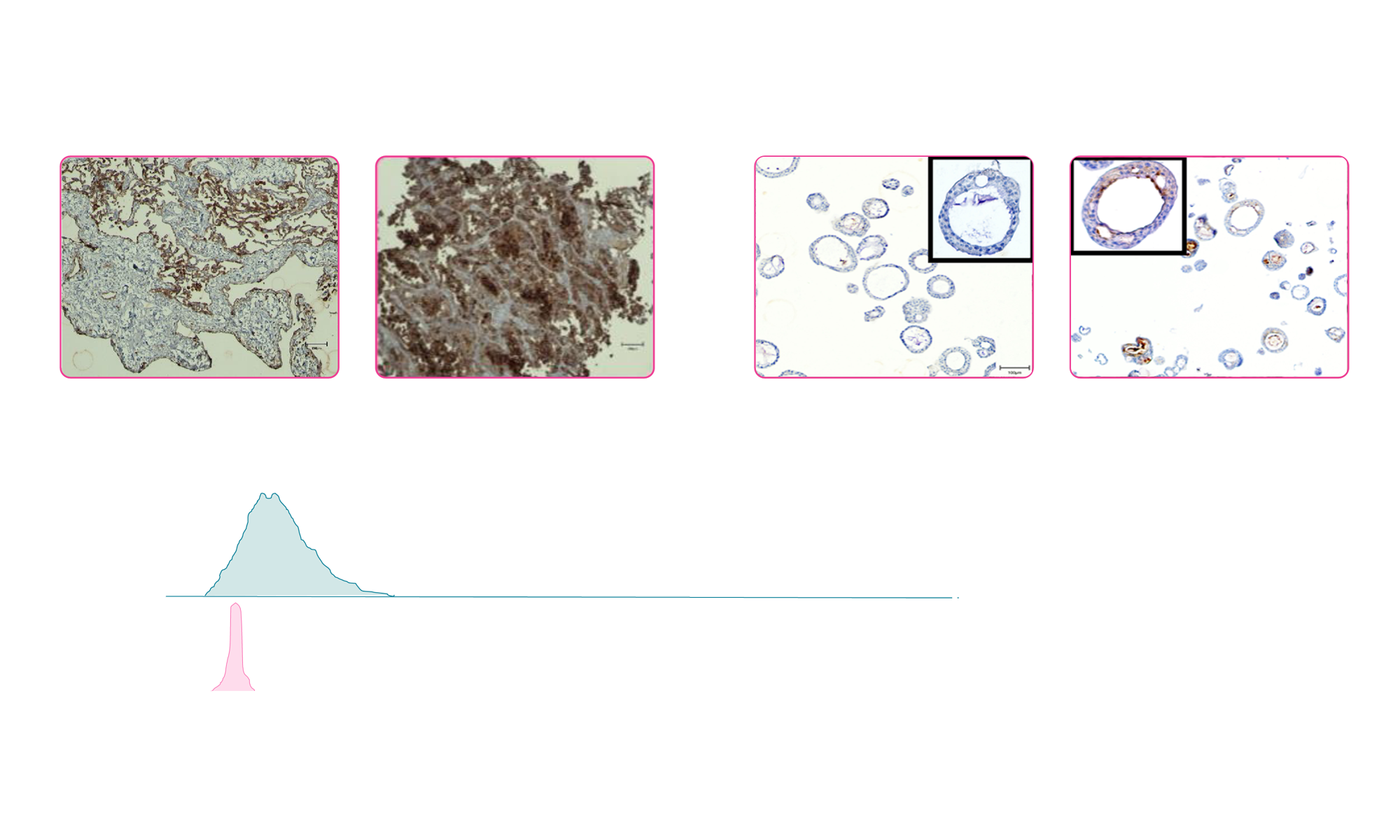
Fig 1: (A) Representative images of IHC staining for the TAA in human tumor tissue and matched normal epithelium and corresponding patient-derived organoids. White arrowheads indicate expression of the TTA in tumor epithelial cells. (B) Flow cytometry analysis of TAA in NSCLC PDO and matched normal lung PDO
By different approaches (ICH and flow cytometry), we confirmed expression of the TAA specifically in the tumor PDOs. This ensured both specificity and the relevance of the chosen models for subsequent efficacy and toxicity testing.
The bispecific antibody (BiTE) induced significant killing of tumor PDOs when co-cultured with T cells. Normal PDOs were largely spared under identical conditions (Figure 2). No cell death occurred in the absence of T cells or when using a non-targeting BiTE (nonspecific), confirming both antigen specificity and T cell dependence.
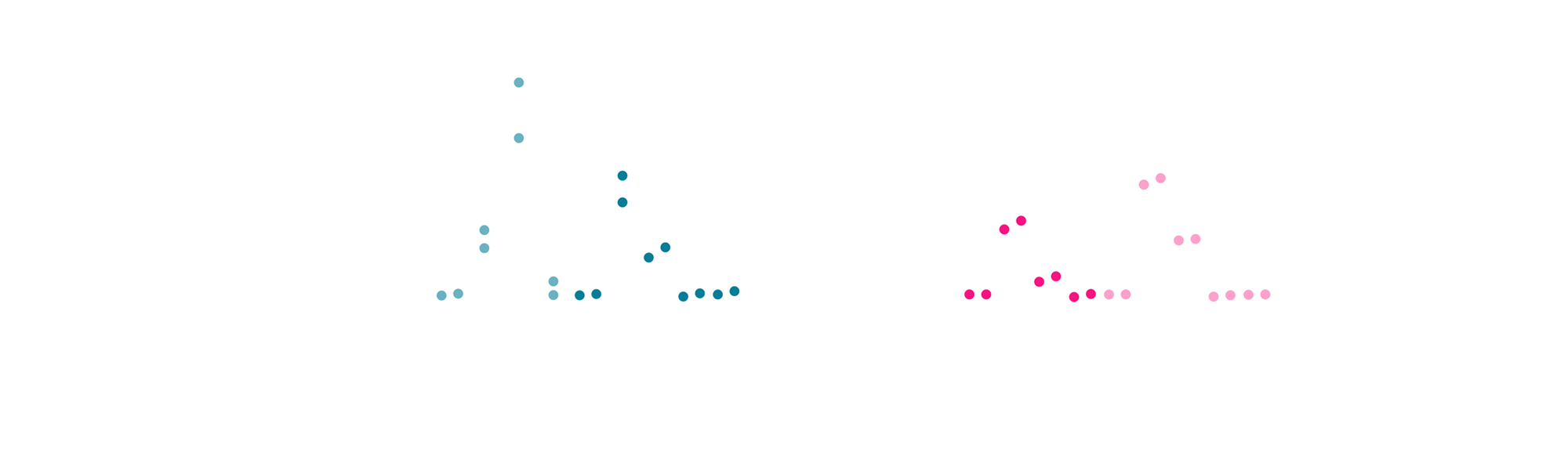
Fig 2: Day 2 quantification of cell death fluorescent signal in A) tumor and B) normal PDOs when exposed to a BiTE directed to a tumor-specific antigen. For organoid killing, staurosporine (STS) and cytokines are used as positive control and medium only as negative control. T cell Trans activator is included as a control for T-cell activation (TnsA). In absence of T- cells, the tested BiTE (TnsA and nonspecific ab) is not able to induce PDO cell death.

IFN-γ secretion was significantly elevated in co-cultures treated with the targeted BiTE, indicating effective activation of T cells in response to TAA recognition on tumor PDOs.

This study demonstrated that the bispecific antibody elicited antigen-specific, T cell–dependent killing of NSCLC PDOs, with minimal off-tumor effects on matched normal organoids. Elevated IFN-γ secretion confirmed functional T cell engagement. The PDO–T cell co-culture system provided insight into the bispecific’s efficacy, and selectivity supporting its progression toward in vivo validation. For bispecific antibody developers, this platform offers a translationally relevant, human-derived system to de-risk immunotherapeutic candidates early in development by modeling tumor–immune dynamics and off-tumor toxicity in vitro.