
Our client, a developer of Antibody-Drug Conjugates (ADCs), wanted to evaluate whether patient-derived organoids could serve as a reliable and translationally relevant platform for ADC development, specifically for biomarker identification and mechanism-of-action studies. To test this, they chose Tivdak™ (tisotumab vedotin) as a representative case. Tivdak™ is an FDA-approved ADC targeting Tissue Factor (TF/CD142), used in the treatment of recurrent or metastatic cervical cancer. Despite its clinical success, Tivdak™ presents a common ADC development challenge: response does not clearly correlate with TF expression levels, making it difficult to predict which patients will benefit from treatment.
Our client wanted to know: Could organoid models replicate this complexity? And more importantly, could they help explain it?

We applied our patient-derived organoid (PDO) platform to evaluate the translational relevance of Tivdak™ and explore potential biomarkers of response. Using 22 tumor organoid models spanning seven indications, we performed an OrganoID-Flow Efficacy Screen to assess treatment response across a range of TF expression levels. TF protein was quantified via FACS and validated against RNAseq data to confirm antigen expression. This setup enabled systematic testing of Tivdak™ across genetically diverse, patient-derived tumor models, allowing our client to investigate correlations between target expression, payload sensitivity, and response. This could not have been achieved using traditional in vitro platforms such as cell lines – which are clonal (i.e. genetically identical) or tumor explants (not amenable to high-throughput screens), thus providing a unique resource for patient-relevant ADC development.

Flow cytometry analysis of TF (target antigen) protein expression across the 22 patient-derived organoid models showed a strong correlation with RNA sequencing data. This finding confirmed that transcriptomic profiling can reliably reflect surface antigen levels in the organoid system. For our client, this established confidence in the use of RNAseq as a scalable proxy for antigen screening and validates the fidelity of the organoid models in maintaining clinically relevant biomarker expression profiles.
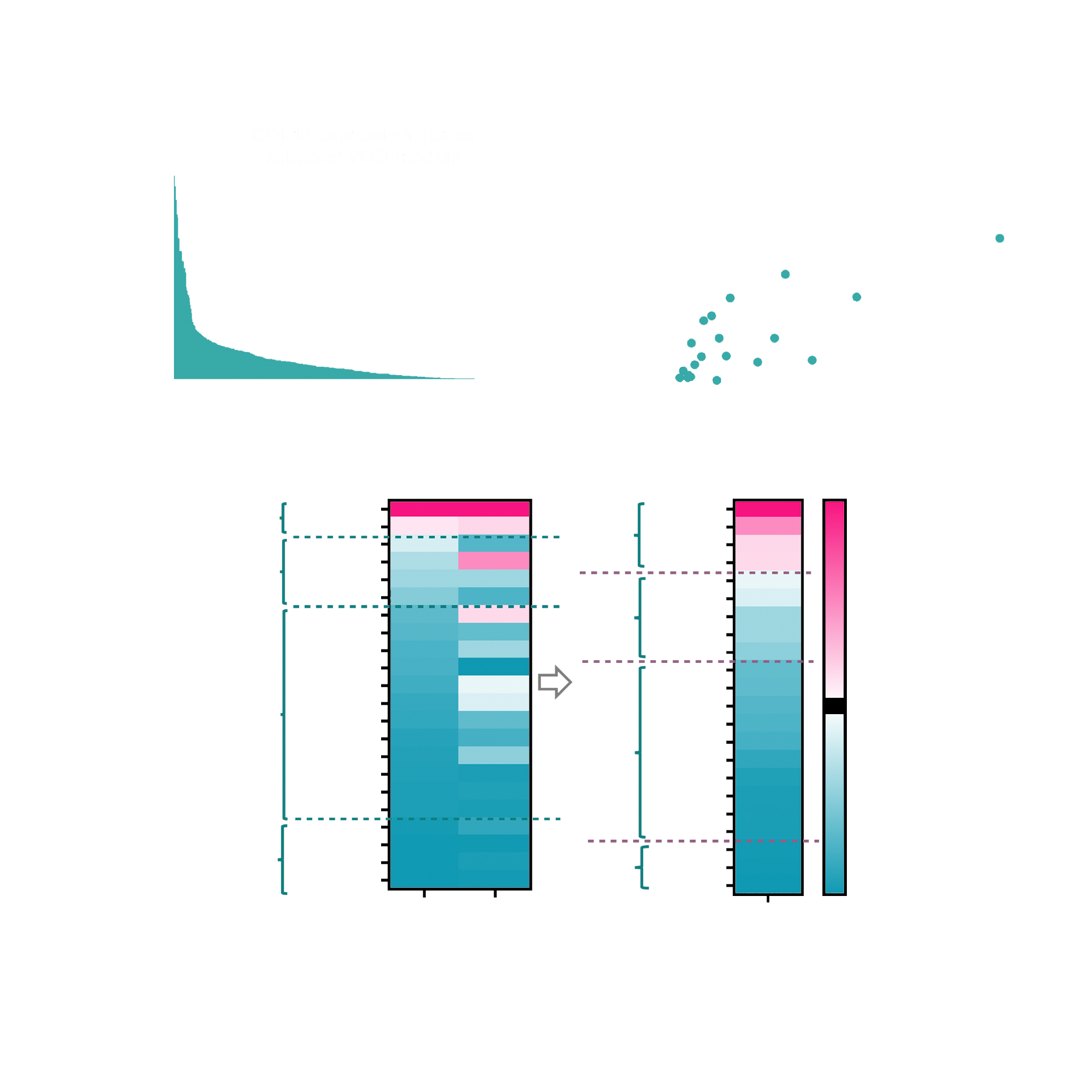
Fig. 1 A) Flow cytometry analysis of CD142 (Tissue Factor) surface expression across 22 patient-derived organoid models representing multiple tumor types. B) Correlation of CD142 protein expression (FACS) with transcript levels derived from RNA sequencing. C) Heatmap classification of organoid models into high, medium, and low CD142 expressers based on integrated FACS and RNAseq data.
Despite clear and quantifiable TF expression across the tested organoid models, there was no consistent correlation with Tivdak™ response. Some organoids with high TF levels showed limited response, while others with lower expression demonstrated notable sensitivity. This lack of correlation aligns with clinical trial observations in cervical cancer patients, where TF expression alone did not reliably predict treatment outcomes. The data confirmed that target expression, while necessary for ADC binding, is not sufficient as a standalone biomarker for predicting efficacy.
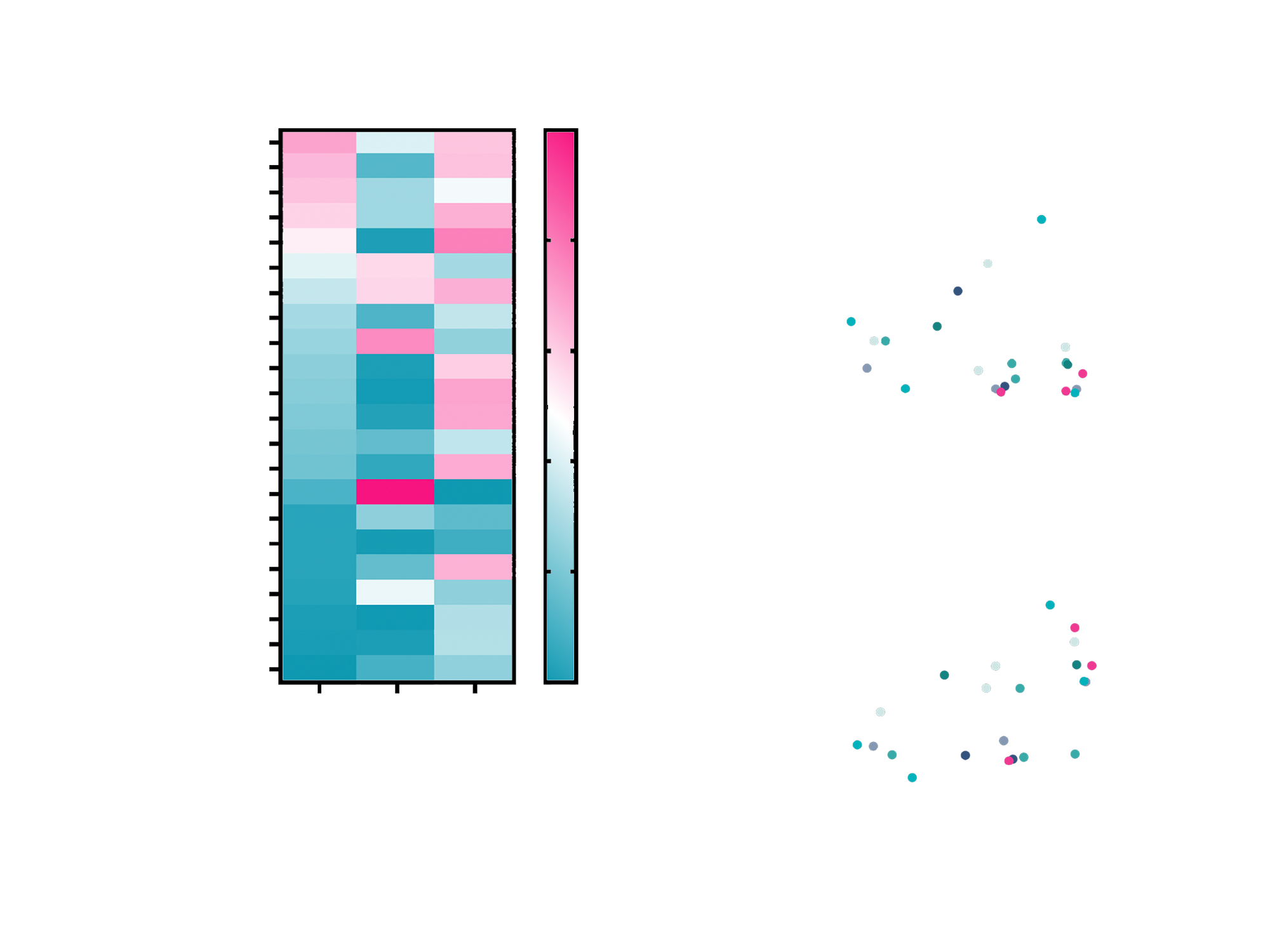
Fig. 2 A) Heatmap summarizing Tivdak™ efficacy across 22 patient-derived organoid models in relation to CD142 (Tissue Factor) protein expression (FACS) and intrinsic sensitivity to MMAE, the cytotoxic payload of Tivdak™. B) Scatter plot showing no correlation between CD142 expression levels and Tivdak™ efficacy (R² = 0.049), indicating that target abundance alone does not predict treatment response. C) Scatter plot demonstrating a moderate correlation between Tivdak™ efficacy and MMAE sensitivity (R² = 0.25), suggesting that intrinsic payload susceptibility is a key driver of response.
Further analysis revealed that variability in Tivdak™ efficacy was better explained by differences in tumor-intrinsic sensitivity to the cytotoxic payload (MMAE). Organoid models exhibited a spectrum of responses independent of TF levels, suggesting that factors such as intracellular trafficking, apoptotic priming, and drug efflux mechanisms may underlie payload susceptibility. For the client, this finding underscored the value of identifying complementary biomarkers related to payload response. It also supported the strategic shift toward dual-biomarker approaches – targeting both antigen presence and intracellular drug sensitivity—to enhance patient selection and ADC design.
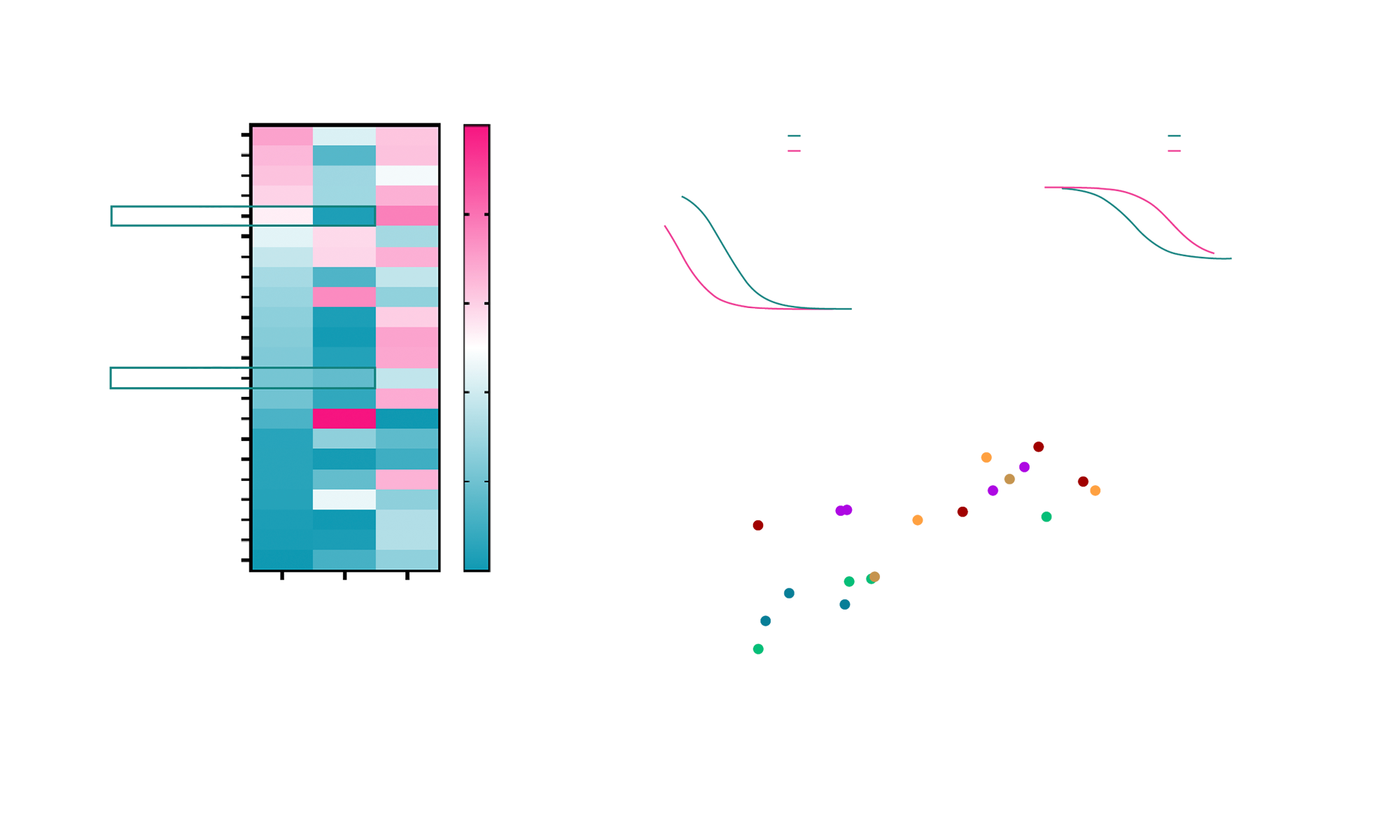
Fig. 3 A) Heatmap summarizing Tivdak™ response across 22 organoid models, annotated with CD142 (Tissue Factor) expression levels (via FACS) and intrinsic sensitivity to MMAE, the payload of Tivdak™. B-C) Representative dose–response curves for Tivdak™ and MMAE in selected organoid models, illustrating variability in response independent of CD142 expression. D) Correlation analysis between CD142 protein expression (mean fluorescence intensity, MFI) and the IC₅₀ ratio of Tivdak™ to MMAE.
This study validated that our patient-derived organoid platform can accurately model the complex biology of ADC response, providing value in both mechanism-of-action research and biomarker strategy development.
For our client, the key takeaways were:
Ultimately, this project demonstrated that organoids can be a reliable, translationally relevant platform for ADC development, de-risking decisions around target selection, payload design, and clinical trial strategy.